Fractional Distillation – How it Works?
The basic idea behind fractional distillation is the same as simple distillation. The difference between simple and fractional distillation is the number of times that the liquid is vaporized and condensed. Simple distillation condenses the liquid once, so the boiling points of the two liquids must be far apart to make it efficient. Simple distillation is performed on a mixture of liquids with similar volatilities and the resulting distillate is in a more concentrated form in the more volatile compound than the original mixture and it may contain a significant amount of the higher boiling compound. If the distillate of simple distillation is distilled again, the resulting distillate is again of a further more concentrated form of the lower boiling compound, but still, a portion of the distillate contains the higher boiling compound. The number of simple distillations in a fractional distillation depends on the length and efficiency of the fractionating column.
Fractional distillation is a process in which vaporization of a liquid mixture gives rise to a mixture of constituents from which the desired one is separated in pure form. This method is also known as rectification because a part of the vapour is condensed and returned as a liquid. This method is used to separate miscible volatile liquids, whose boiling points are close, using a fractionating column. Fractional distillation is different from simple distillation. In simple distillation, the vapour is directly passed through the condenser. In fractional distillation, the vapour must pass through a fractionating column in which partial condensation of vapour is allowed to occur. In simple distillation, condensate is collected directly into the receiver, while in fractional distillation condensation takes place in the fractionating column so that a part of the condensing vapour returns to the still.
HomePharmaceutical Engineering
Fractional Distillation – How it Works?
The basic idea behind fractional distillation is the same as simple distillation. The difference between simple and fractional distillation is the number of times that the liquid is vaporized and condensed. Simple distillation condenses the liquid once, so the boiling points of the two liquids must be far apart to make it efficient. Simple distillation is performed on a mixture of liquids with similar volatilities and the resulting distillate is in a more concentrated form in the more volatile compound than the original mixture and it may contain a significant amount of the higher boiling compound. If the distillate of simple distillation is distilled again, the resulting distillate is again of a further more concentrated form of the lower boiling compound, but still, a portion of the distillate contains the higher boiling compound. The number of simple distillations in a fractional distillation depends on the length and efficiency of the fractionating column.

Fractional distillation is a process in which vaporization of a liquid mixture gives rise to a mixture of constituents from which the desired one is separated in pure form. This method is also known as rectification because a part of the vapour is condensed and returned as a liquid. This method is used to separate miscible volatile liquids, whose boiling points are close, using a fractionating column. Fractional distillation is different from simple distillation. In simple distillation, the vapour is directly passed through the condenser. In fractional distillation, the vapour must pass through a fractionating column in which partial condensation of vapour is allowed to occur. In simple distillation, condensate is collected directly into the receiver, while in fractional distillation condensation takes place in the fractionating column so that a part of the condensing vapour returns to the still.
Principle of Fractional Distillation
When a liquid mixture is distilled, the partial condensation of the vapours is allowed to occur in a fractionating column. In the column, ascending vapours from the still are allowed to come in contact with the condensing vapours returning to the still. This result is an enrichment of the vapours with the more volatile component. By condensing the vapours and reheating the liquid repeatedly, equilibrium between liquid and vapours is set up at each stage, which ultimately results in the separation of a more volatile component.
Construction of Fractional Distillation
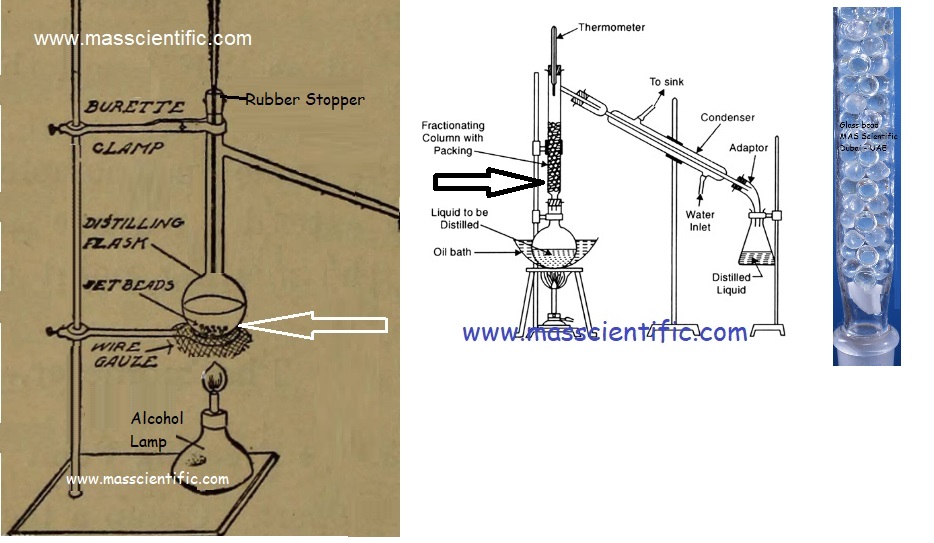
The equipment used for fractional distillation consists of a special type of still-heads known as fractionating columns. In still-heads condensation and revapourisation are affected continuously. A fractionating column is essentially a long vertical tube in which the vapours moves upward and partially gets condensed. The condensate flows down the column and is returned to the flask. The columns are constructed to provide a large cooling surface for the vapours to condense and obstruct the ascending vapour to allow easy condensation. The obstruction also retards the downward flow of liquid, which is a high boiling component. Fractionating columns used are packed columns and plate columns.
Packed columns: In this column, some form of packing is used to affect the necessary liquid/vapour contact. The packing consists of single turn helices (spirals) of wire or glass, glass rings, cylindrical glass beads, stainless steel rings etc. The column consists of a tower containing a packing that becomes wetted with a film of liquid, which is brought into contact with the vapours in the intervening spaces. The same type of fractionating columns can be obtained in various lengths. A long fractionating column is necessary when the boiling points of the constituents are lying fairly close together. A short fractionating column is necessary when the boiling point of the constituents differ considerably. Packing must be uniform to obtain proper channels. If packing is irregular, the mass transfer becomes less effective. An example is the Widmer column.
MAS Scientific lab Dev Tr LLC, Dubai, Explain the procedure for Scientific Laboratory Users.
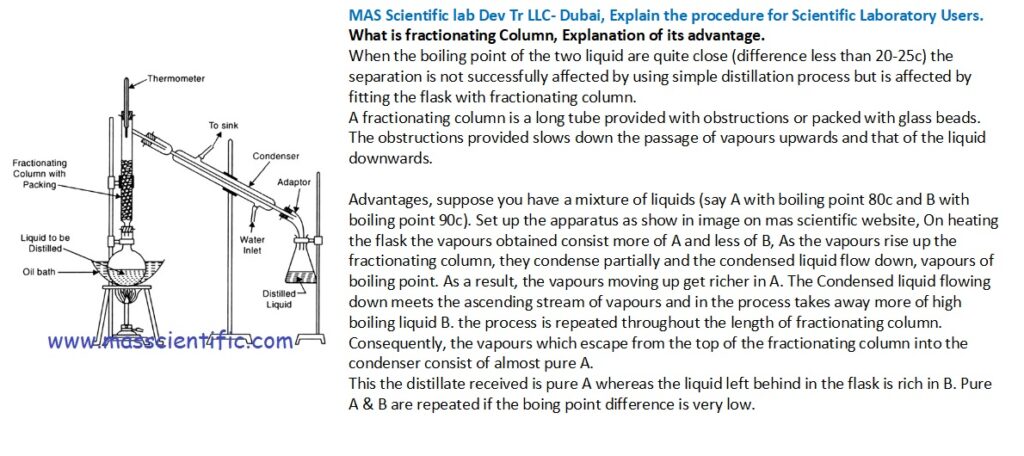
What is fractionating Column, Explanation of its advantage.
When the boiling point of the two liquid are quite close (difference less than 20-25c) the separation is not successfully affected by using simple distillation process but is affected by fitting the flask with fractionating column.
A fractionating column is a long tube provided with obstructions or packed with glass beads.
The obstructions provided slows down the passage of vapours upwards and that of the liquid downwards.
Advantages, suppose you have a mixture of liquids (say A with boiling point 80c and B with boiling point 90c). Set up the apparatus as show in image on mas scientific website, On heating the flask the vapours obtained consist more of A and less of B, As the vapours rise up the fractionating column, they condense partially and the condensed liquid flow down, vapours of boiling point. As a result, the vapours moving up get richer in A. The Condensed liquid flowing down meets the ascending stream of vapours and in the process takes away more of high boiling liquid B. the process is repeated throughout the length of fractionating column. Consequently, the vapours which escape from the top of the fractionating column into the condenser consist of almost pure A.
This the distillate received is pure A whereas the liquid left behind in the flask is rich in B. Pure A & B are repeated if the boing point difference is very low.